Black Box Warnings: What Every Nurse Should Know
Black Box Warnings: Just the words sound serious. And they are. The U.S. Food and Drug Administration (FDA) requires a Black Box Warning (also called a black label warning or boxed warning) to be placed on medications that carry critical safety risks. It’s the FDA’s strictest labeling requirements that the FDA can mandate for prescription drugs.
The name comes from the black border surrounding the text of the warning that appears on the package insert, label, and other information, such as a magazine advertisement. A Black Box Warning must provide an accurate and concise summary of the drug’s adverse effects and the risks associated when taking the medication, even when taken as prescribed.
All prescription medications must be approved by the FDA. Approval comes only after rigorous studies that can document safe and effective use. In a perfect world, all possible adverse reactions would already be known before being approved and prescribed. But once a drug is available to a wide population, unexpected serious side effects can occur. This is when the FDA requires a Black Box Warning.
What situations require a Black Box Warning?
Since 1979, the FDA has required Black Box Warning for these circumstances:
- A medication can cause serious, permanently disabling, life-threatening, or fatal effects, compared to the potential benefit of the drug. The health care provider and the patient should decide together if the medication is worth the risk.
- Serious adverse reactions can possibly be prevented or reduced in severity by proper administration and monitoring in a defined population. Monitoring lab values and avoiding drug interactions may be necessary.
- Restricting administration to certain groups to ensure safe use. Examples: NOT for use during pregnancy, in children or the elderly.
What does the label look like?

The FDA wants patients and healthcare providers to see any warnings right away. The box contains a summary of adverse effects and risks that have been associated with the drug.
To draw attention, alerts always have the following:
- The Black Box Warning is prominently placed at the TOP of the drug insert.
- It’s enclosed by a black border.
- All the words are in BOLD print.
- The warning may also be placed on the prescription bottle.
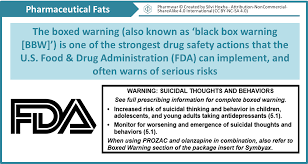
Here is the wording for sertraline (Zoloft), a selective serotonin reuptake inhibitor (SSRI) antidepressant:
Suicidality in Children and Adolescents
Antidepressants increased the risk of suicidal thinking and behavior (suicidality) in short-term studies in children and adolescents with Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of Zoloft or any other antidepressant in a child or adolescent must balance this risk with the clinical need. Patients who are started on therapy should be observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Zoloft is not approved for use in pediatric patients except for patients with obsessive-compulsive disorder (OCD).
What medications have it?
Over 400 medications have Black Box Warnings. Usually, the boxed warning applies to a class of drug, rather than individual medications.
Common medications that must include a Black Box Warning include:
- ACE inhibitors: Angiotensis converting enzyme (ACE) inhibitors treat hypertension and certain heart conditions. They can cause harm to a fetus and should not be taken during pregnancy. Examples: lisinopril (Prinvil, Zestril) and enalapril (Vasotec).
- Antidepressants: Although antidepressants are important in treating depression and other mental health conditions, they can increase the risk of suicidal thoughts and behaviors. Children and young adults under the age of 25 are at a higher risk. Classes of antidepressants include selective serotonin reuptake inhibitors (SSRI), serotonis norepinephrine reuptake inhibitors (SNRI) and tricyclic antidepressants (TCA).
- Combination birth control pills: Birth control pills that contain both estrogen and progestin can increase the risk of blood clots, which can cause a heart attack or stroke. Risk increases even more for females over age 35 and smokers. Examples: norethindrone/ethinyl estradiol/ferrous fumarate (Loestrin FE 1/20) and drospirenone/ethinyl estradiol (Yaz).
- Fluoroquinolone antibiotics: These antibiotics have an increased risk of tendinitis and tendon rupture, an acute injury that can result in permanent disability. Examples: ciprofloxacin (Cipro), levofloxacin (Levaquin), and moxifloxacin (Avelox).
- Glitazones: Glitazones are a class of drugs used to treat Type 2 diabetes. They can worsen congestive heart failure in people with existing symptomatic CHF, and should be avoided. Examples: pioglitazone (Actos) and rosiglitazone (Avandia).
- NSAIDS: Non-steroidal anti-inflammatory drugs (NSAIDs) are useful in managing mild to moderate pain. However, they can raise the risk of a heart attack or stroke, especially for people with current heart conditions or risk factors for heart disease, including a recent heart attack. They also increase the risk for gastrointestinal problems, including ulcers and GI bleeding. Examples: naproxen (Aleve, Naprosyn) and ibuprofen (Advil, Motrin).
- Opioids: The FDA mandates Black Box Warnings on all opioid medications (immediate-acting, extended-release, and long-acting) that emphasize the risk of misuse, abuse, dependence, overdose, and death—even at recommended dosages. The FDA also stresses that opioids are to be used only in cases of severe pain, under close supervision of a physician.
What are the roles and responsibilities of health professionals?
The National Council of State Boards of Nursing (NCSBN) is the organization that oversees the NCLEX-RN. In the Test Plan for the RN licensure examination, one of the responsibilities of a Registered Nurse is to “handle and administer high-risk medications.”
The Nursing “Rights” of Medication Administration are always important, and with Black Box drugs, they are critical. The nurse is the FINAL person to verify that the patient is receiving the correct drug and dose. The traditional “Five Rs” are:
- Right patient: The nurse is administering the prescribed medication to the correct recipient.
- Right drug: The medication that the nurse will administer is identical to the drug name that has been prescribed.
- Right route: The nurse should know the bioavailability of the drug to ensure it corresponds to the prescribed method of administration.
- Right time: The medication is administered at the specific prescribed interval to maintain a therapeutic level.
- Right dose: The nurse must verify that the prescribed dose is appropriate for the patient.
Although following the Rights of Medication Administration is essential, the nurse must also use critical thinking to avoid medication errors and keep patients safe. Every nurse should stay updated on the new medications that are most commonly prescribed for patients on their hospital unit or in their specialty. Reading the package insert is important, no matter the level of nursing experience. A Black Box Warning will be in a prominent place; other information regarding dosage, adverse effects, and contraindications will also be provided.
Registered Nurses are also expected to be aware of possible drug interactions. If you’re unfamiliar with a medication, take time to find out if it’s compatible with any other medications that your patient is currently taking. Contact the pharmacist or look up the drug online. Don’t administer a medication without checking if it can harm the patient. If in doubt, notify the health care provider who wrote the prescription.
Patient teaching
Whenever possible, patients should always be included in their own health care. When they are able to understand the purpose of their medication, as well as possible side effects, they can become competent consumers who can participate in decision making.
The nurse is often responsible for educating a patient about their medications at the time of hospital discharge or after an office/clinic appointment. It’s helpful is another person also receives the information to avoid future confusion.
Teaching points to remember include:
- Focus on the fact that a Black Box drug requires special attention.
- The medication has risks, but it has benefits that make it worthwhile for this patient.
- If the warning has serious adverse reactions that apply to the patient, make sure the patient has had the opportunity to discuss the drug with the prescriber. If not, contact the prescriber to talk with the patient.
- Remind the patient that their pharmacist can answer questions about warnings.
- After explaining the purpose of the Black Box drug, ask the patient to tell you about it in their own words.
- Stress the adverse effects that this particular patient may experience, but also a general range.
- Instruct the patient what to do if they have an adverse reaction. Some medications should be stopped immediately, but others must be weaned. Ask the patient (and other supporting person) to verbalize the instruction.
- Review all other medication instructions: dosage, schedule, storage, etc.
- Ask the patient to demonstrate how to self-administer the medication. Check for proper preparation, dosage, and technique. Correct and reinforce as necessary.
- Tell the patient to notify their health care provider if they stop or start any of their other medications, whether prescribed or over-the-counter (OTC).
- Document the teaching session in the patient’s health record.
Medications are always a team effort
While the nurse is the final step in administering medications, each person on the interdisciplinary team has a responsibility. The prescriber should know their patient and the medication well enough to determine if a drug with a Black Box Warning is the best option. Pharmacists should take every precaution to ensure that the drug is properly prepared and labeled, with exact instructions and warnings. The nurse double-checks everything before administering or providing patient education. Even the patient has a responsibility to ask questions and demonstrate understanding before self-administration.
 By
By 



